AOD-9604 (5MG)
$47.00 Original price was: $47.00.$42.00Current price is: $42.00.
Contents: Lyophilized Powder in 3ml vial
Requires reconstitution with bacteriostatic water
LABORATORY HANDLING NOTES: AOD-9604 has unique solubility characteristics that researchers should be aware of when preparing solutions for in vitro applications.
Recommended Solvent Protocol: This compound demonstrates optimal solubility when reconstituted using a combination of 0.6% acetic acid solution and sterile water. Add solvents gradually along the vial wall to minimize agitation, as excessive mechanical disruption may affect solution clarity.
Solution Stability: Due to the high purity of this preparation, reconstituted AOD-9604 may exhibit increased viscosity or gel formation over time. This is a known characteristic of highly concentrated AOD-9604 solutions and does not indicate degradation. Researchers should prepare working aliquots promptly after reconstitution for optimal handling.
Note: If increased viscosity occurs, additional acetic acid solution may restore fluidity.
Rigorous Third-Party Testing
Every batch of our research chemicals and peptides undergoes third-party testing.
- Description
- 3rd Party Lab Testing
Description
AOD-9604
AOD-9604 is a synthetic hexadecapeptide corresponding to an hGH C-terminal fragment (residues 176–191) with an N-terminal tyrosine substitution. It is supplied as a defined peptide sequence for laboratory studies involving peptide characterization, stability profiling, and structure–function investigation of growth-hormone–derived fragments.
Product Specifications
| Sequence | H-Tyr-Leu-Arg-Ile-Val-Gln-Cys(1)-Arg-Ser-Val-Glu-Gly-Ser-Cys(1)-Gly-Phe-OH (YLRIVQCRSVEGSCGF; disulfide between Cys residues) |
| Molecular Formula | C78H123N23O23S2 |
| Molecular Weight | 1815.10 g/mol |
| CAS Number | 221231-10-3 |
| Purity | ≥98% (HPLC verified) |
| Form | Lyophilized powder |
| Appearance | White to off-white powder |
Research Applications
- Structure–activity relationship (SAR) workflows evaluating hGH-fragment sequence features and disulfide-dependent conformational constraints
- Peptide identity and purity confirmation using LC–MS and/or LC–MS/MS (intact mass, peptide mapping, and impurity profiling)
- Peptide stability and degradation analysis under buffered aqueous conditions (pH stress, oxidation, deamidation, and forced-degradation panels)
- In vitro pathway screening using standardized cell-based assay formats (e.g., reporter assays, phosphorylation readouts, or second-messenger panels) where growth-hormone–derived fragments are used as test articles
- Protein/peptide interaction testing in vitro (e.g., nonspecific binding/adsorption studies, carrier-protein interaction checks, and assay-surface compatibility screening)
Quality Documentation
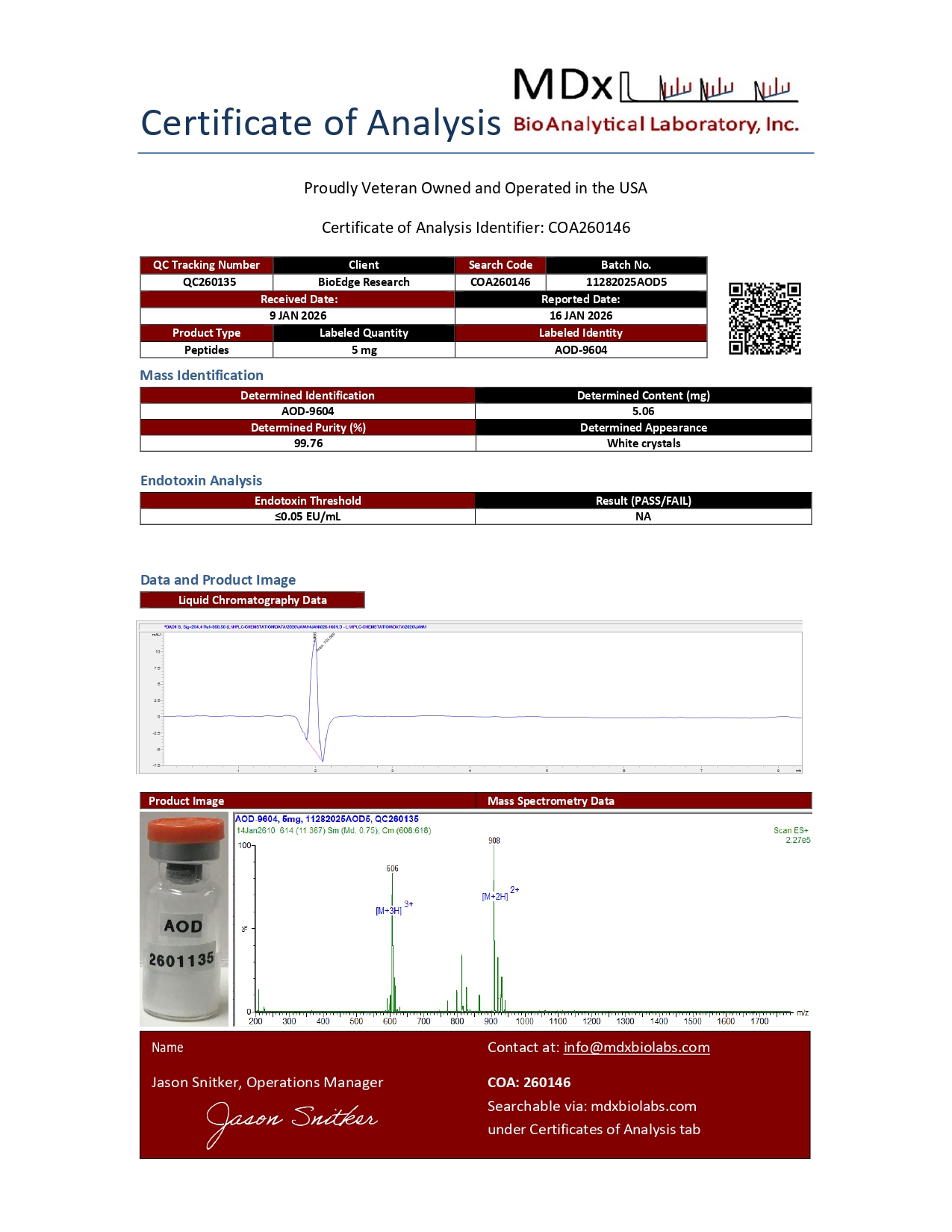
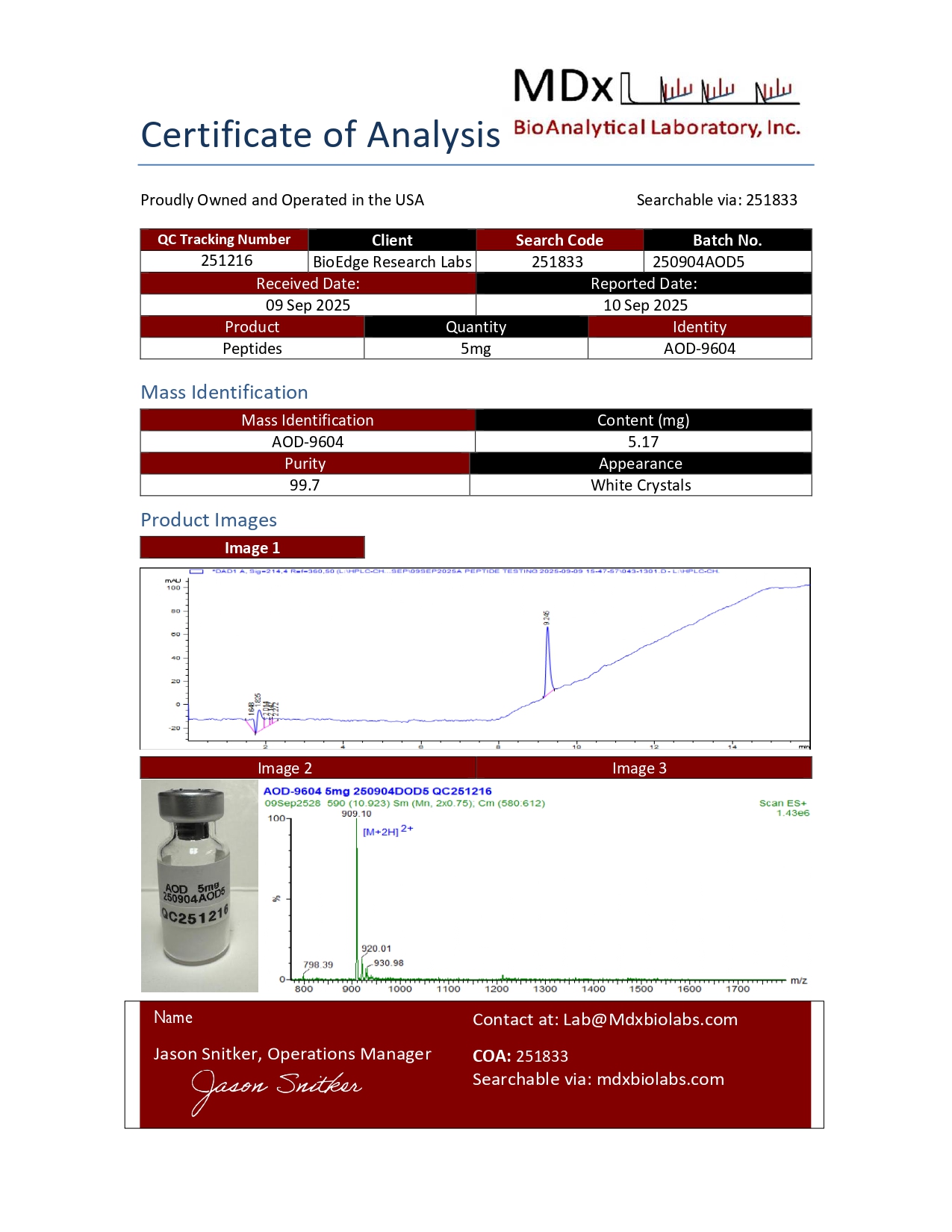
Certificate of Analysis and HPLC chromatogram available for each lot.
For Laboratory Research Use Only
This product is intended exclusively for in vitro laboratory research. Not for human or veterinary use. Not for diagnostic, therapeutic, or clinical applications.